The Major Factors Affecting Oocyte Quality in IVF Cycles: A Narrative Review
DOI:
https://doi.org/10.38179/ijcr.v3i1.277Keywords:
Oocyte Quality, IVF cycle, Obesity, Age, Endometriosis, PCOS, Cyclic Nucleotides, Thalassemia, Markers, MalignanciesAbstract
Background: The role of assisted reproductive technologies, including in-vitro fertilization [IVF], is increasing daily because of the significant rise in subfertility cases. Among women, the most observed causes of infertility are primary ovarian insufficiency and premature ovarian failure, accounting for 25% of cases, followed by tubal damage (20%) and uterine abnormalities (10%), all contributing to the increase in IVF cases among couples. The success of IVF depends on various factors; however, the role of oocyte quality and maturation level is considered a pivotal cardinal factor for the success rates of IVF.
Methods: A thorough literature analysis was performed using the following search terms: “Oocyte Quality” and “IVF cycle.” The databases searched included PubMed, Google Scholar, MEDLINE, Cochrane Library, and ResearchGate.
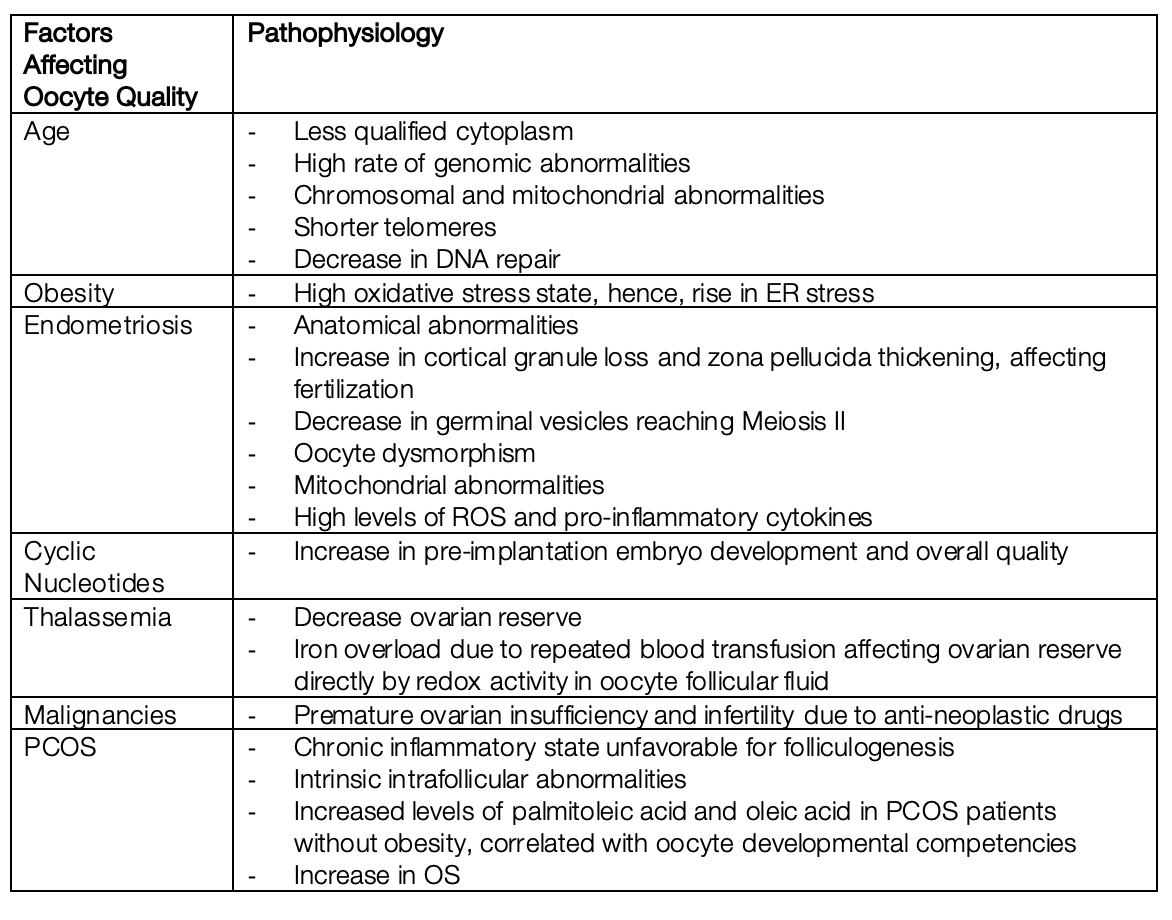
Discussion: IVF success rates especially depend on oocyte quality and level of maturation. Several factors affecting these two factors include obesity, which increases O2 and H2O2 levels resulting in elevated endoplasmic reticulum [ER] stress; Polycystic Ovary Syndrome [PCOS]; age; endometriosis; cyclic nucleotides used in IVF; thalassemia major which is associated with lower ovarian reserve and increased redox activity malignancies, and anti-neoplastic drugs, which may contribute to premature ovarian insufficiency. Various treatment options were proposed to improve oocyte quality and maturation level, including growth hormone [GH] supplementation alongside ovarian supplementation, autologous mitochondrial transfer, luteal phase ovarian stimulation, administration of MI-Melatonin-Vitamin D3, Duphaston, and putrescine supplementation.
Conclusion: With the rising number of subfertility cases, the importance of Assisted Reproductive Technologies [ART] is growing. The success rate of IVF on oocyte quality and level of maturation level, and few with several factors affecting these. Though numerous treatment options have been proposed to enhance oocyte quality and maturation, not all have been deemed beneficial.
References
Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980 September;879:737–56. PMID: 6775685. https://doi.org/10.1111/j.1471-0528.1980.tb04610.x.
Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005 May;205:1144–7. PMID: 15802321. https://doi.org/10.1093/humrep/deh870.
Gaffney, T. (2023, April 4). 1 in 6 people globally face infertility at some point in life, who says. PBS. https://www.pbs.org/newshour/health/1-in-6-people-globally-face-infertility-at-some-point-in-life-who-says#
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992 July 4;3408810:17–8. PMID: 1351601. https://doi.org/10.1016/0140-6736(92)92425-f.
Yeste M, Jones C, Amdani SN, Patel S, Coward K. Oocyte activation deficiency: a role for an oocyte contribution? Hum Reprod Update. 2016 February;221:23–47. PMID: 26346057. https://doi.org/10.1093/humupd/dmv040.
Chen C-H, Tzeng C-R, Wang P-H, et al. Dual triggering with GnRH agonist plus hCG versus triggering with hCG alone for IVF/ICSI outcome in GnRH antagonist cycles: a systematic review and meta-analysis. Arch Gynecol Obstet. 2018 July;2981:17–26. PMID: 29600322. https://doi.org/10.1007/s00404-018-4751-3.
Huddleston HG, Jackson KV, Doyle JO, Racowsky C. hMG increases the yield of mature oocytes and excellent-quality embryos in patients with a previous cycle having a high incidence of oocyte immaturity. Fertil Steril. 2009 September;923:946–9. PMID: 19356754. https://doi.org/10.1016/j.fertnstert.2009.02.039.
Lee HJ, Jee BC, Suh CS, Kim SH, Moon SY. Oocyte maturity in relation to woman’s age in vitro fertilization cycles stimulated by a single regimen. Yonsei Med J. 2012 January;531:181–5. PMID: 22187250. https://doi.org/10.3349/ymj.2012.53.1.181.
Vitek WS, Shayne M, Hoeger K, Han Y, Messing S, Fung C. Gonadotropin-releasing hormone agonists for the preservation of ovarian function among women with breast cancer who did not use tamoxifen after chemotherapy: a systematic review and meta-analysis. Fertil Steril. 2014 Septem-ber;1023:808-815.e1. PMID: 25044080. https://doi.org/10.1016/j.fertnstert.2014.06.003.
van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005 April;834:979–87. PMID: 15820810. https://doi.org/10.1016/j.fertnstert.2004.11.029.
Weenen C, Laven JSE, Von Bergh ARM, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004 February;102:77–83. PMID: 14742691. https://doi.org/10.1093/molehr/gah015.
Pacchiarotti A, Iaconianni P, Caporali S, et al. Severe endometriosis: low value of AMH did not affect oocyte quality and pregnancy outcome in IVF patients. Eur Rev Med Pharmacol Sci. 2020 No-vember;2422:11488–95. PMID: 33275215. https://doi.org/10.26355/eurrev_202011_23790.
Dai X, Wang Y, Yang H, et al. AMH has no role in predicting oocyte quality in women with advanced age undergoing IVF/ICSI cycles. Sci Rep. 2020 November 12;101:19750. PMID: 33184364. https://doi.org/10.1038/s41598-020-76543-y.
McKnight K, McKenzie LJ. Evaluation of Infertility, Ovulation Induction and Assisted Reproduction. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext, South Dartmouth (MA): MDText.com, Inc.; 2000.
Qiao J, Wang Z-B, Feng H-L, et al. The root of reduced fertility in aged women and possible therapeutic options: current status and future perspects. Mol Aspects Med. 2014 August;38:54–85. PMID: 23796757. https://doi.org/10.1016/j.mam.2013.06.001.
May-Panloup P, Boucret L, Chao de la Barca J-M, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016 November;226:725–43. PMID: 27562289. https://doi.org/10.1093/humupd/dmw028.
Cheng J-M, Liu Y-X. Age-Related Loss of Cohesion: Causes and Effects. Int J Mol Sci. 2017 July 22;187:E1578. PMID: 28737671. https://doi.org/10.3390/ijms18071578.
Zhang J-J, Liu X, Chen L, et al. Advanced maternal age alters expression of maternal effect genes that are essential for human oocyte quality. Aging (Albany NY). 2020 February 25;124:3950–61. PMID: 32096767. https://doi.org/10.18632/aging.102864.
Menezo Y, Russo G, Tosti E, El Mouatassim S, Benkhalifa M. Expression profile of genes coding for DNA repair in human oocytes using pangenomic microarrays, with a special focus on ROS linked de-cays. J Assist Reprod Genet. 2007 November;2411:513–20. PMID: 17899356. https://doi.org/10.1007/s10815-007-9167-0.
Schatten H, Sun Q-Y, Prather R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod Biol Endocrinol. 2014 November 24;12:111. PMID: 25421171. https://doi.org/10.1186/1477-7827-12-111.
Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. 2019 September;1583:R79–90. PMID: 30999278. https://doi.org/10.1530/REP-18-0583.
Pohlmeier WE, Xie F, Kurz SG, Lu N, Wood JR. Progressive obesity alters the steroidogenic re-sponse to ovulatory stimulation and increases the abundance of mRNAs stored in the ovulated oo-cyte. Mol Reprod Dev. 2014 August;818:735–47. PMID: 24824196. https://doi.org/10.1002/mrd.22342.
Xie F, Anderson CL, Timme KR, Kurz SG, Fernando SC, Wood JR. Obesity-Dependent Increases in Oocyte mRNAs Are Associated With Increases in Proinflammatory Signaling and Gut Microbial Abun-dance of Lachnospiraceae in Female Mice. Endocrinology. 2016 April;1574:1630–43. PMID: 26881311. https://doi.org/10.1210/en.2015-1851.
Kawwass JF, Kulkarni AD, Hipp HS, Crawford S, Kissin DM, Jamieson DJ. Extremities of body mass index and their association with pregnancy outcomes in women undergoing in vitro fertilization in the United States. Fertil Steril. 2016 December;1067:1742–50. PMID: 27666564. https://doi.org/10.1016/j.fertnstert.2016.08.028.
Luzzo KM, Wang Q, Purcell SH, et al. High-fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;711:e49217. PMID: 23152876. https://doi.org/10.1371/journal.pone.0049217.
Guzel E, Arlier S, Guzeloglu-Kayisli O, et al. Endoplasmic Reticulum Stress and Homeostasis in Re-productive Physiology and Pathology. Int J Mol Sci. 2017 April 8;184:E792. PMID: 28397763. https://doi.org/10.3390/ijms18040792.
Sutton-McDowall ML, Wu LLY, Purdey M, et al. Nonesterified Fatty Acid-Induced Endoplasmic Reticulum Stress in Cattle Cumulus Oocyte Complexes Alters Cell Metabolism and Developmental Competence. Biol Reprod. 2016 January;941:23. PMID: 26658709. https://doi.org/10.1095/biolreprod.115.131862.
Wu LL-Y, Dunning KR, Yang X, et al. The high-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010 November;15111:5438–45. PMID: 20861227. https://doi.org/10.1210/en.2010-0551.
RL;, W. L. D. R. (n.d.). Endoplasmic Reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Molecular endocrinology (Baltimore, Md.). PMID: 22383462. https://doi.org/10.1210/me.2011-1362
Senapati S, Sammel MD, Morse C, Barnhart KT. Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the Society for Assisted Reproductive Technologies Database. Fertil Steril. 2016 July;1061:164-171.e1. PMID: 27060727. https://doi.org/10.1016/j.fertnstert.2016.03.037.
Xu B, Guo N, Zhang X, et al. Oocyte quality is decreased in women with minimal or mild endo-metriosis. Sci Rep. 2015 May 29;5:10779. PMID: 26022105. https://doi.org/10.1038/srep10779.
Nunes JPS, Andrieux P, Brochet P, et al. Co-Exposure of Cardiomyocytes to IFN-γ and TNF-α Induc-es Mitochondrial Dysfunction and Nitro-Oxidative Stress: Implications for the Pathogenesis of Chron-ic Chagas Disease Cardiomyopathy. Front Immunol. 2021;12:755862. PMID: 34867992. https://doi.org/10.3389/fimmu.2021.755862.
Ceviren. Characteristic cytoplasmic morphology of oocytes in endometriosis patients and its effect on the outcome of assisted reproduction treatments cycles n.d. http://www.ivflite.org/article.asp?issn=2348-2907;year=2014;volume=1;issue=2;spage=88;epage=93;aulast=Ceviren (accessed September 11, 2022).
Barcelos ID, Vieira RC, Ferreira EM, Martins WP, Ferriani RA, Navarro PA. Comparative analysis of the spindle and chromosome configurations of in vitro-matured oocytes from patients with endo-metriosis and control subjects: a pilot study. Fertil Steril. 2009 November;925:1749–52. PMID: 19523612. https://doi.org/10.1016/j.fertnstert.2009.05.006.
Dib LA, Araújo MCPM, Giorgenon RC, Romão GS, Ferriani RA, Navarro PA. Noninvasive imaging of the meiotic spindle of in vivo matured oocytes from infertile women with endometriosis. Reprod Sci. 2013 April;204:456–62. PMID: 22991379. https://doi.org/10.1177/1933719112459217.
Fragouli E, Spath K, Alfarawati S, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015 June;116:e1005241. PMID: 26039092. https://doi.org/10.1371/journal.pgen.1005241.
Sanchez AM, Vanni VS, Bartiromo L, et al. Is the oocyte quality affected by endometriosis? A re-view of the literature. J Ovarian Res. 2017 July 12;101:43. PMID: 28701212. https://doi.org/10.1186/s13048-017-0341-4.
Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002 June;776:1148–55. PMID: 12057720. https://doi.org/10.1016/s0015-0282(02)03112-6.
Yang C, Geng Y, Li Y, Chen C, Gao Y. Impact of ovarian endometrioma on ovarian responsiveness and IVF: a systematic review and meta-analysis. Reprod Biomed Online. 2015 July;311:9–19. PMID: 25982092. https://doi.org/10.1016/j.rbmo.2015.03.005.
Harada M, Takahashi N, Hirata T, Koga K, Fujii T, Osuga Y. Laparoscopic excision of ovarian endo-metrioma does not exert a qualitative effect on ovarian function: insights from in vitro fertilization and single embryo transfer cycles. J Assist Reprod Genet. 2015 May;325:685–9. PMID: 25758989. https://doi.org/10.1007/s10815-015-0457-7.
Murphy AA, Santanam N, Morales AJ, Parthasarathy S. Lysophosphatidyl Choline, a Chemotactic Factor for Monocytes/T-Lymphocytes Is Elevated in Endometriosis. The Journal of Clinical Endocri-nology & Metabolism. 1998 June;836:2110–3. https://doi.org/10.1210/jcem.83.6.4823.
Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in en-dometriosis-associated infertility. Fertility and Sterility. 2008 August;902:247–57. https://doi.org/10.1016/j.fertnstert.2008.02.093.
Carvalho LFP, Samadder AN, Agarwal A, Fernandes LFC, Abrão MS. Oxidative stress biomarkers in patients with endometriosis: systematic review. Arch Gynecol Obstet. 2012 October;2864:1033–40. https://doi.org/10.1007/s00404-012-2439-7.
Da Broi MG, de Albuquerque FO, de Andrade AZ, Cardoso RL, Jordão Junior AA, Navarro PA. In-creased concentration of 8-hydroxy-2′-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016 October;3661:231–42. https://doi.org/10.1007/s00441-016-2428-4.
Mansour G, Sharma RK, Agarwal A, Falcone T. Endometriosis-induced alterations in mouse meta-phase II oocyte microtubules and chromosomal alignment: a possible cause of infertility. Fertil Ste-ril. 2010 October;945:1894–9. PMID: 19896655. https://doi.org/10.1016/j.fertnstert.2009.09.043.
Prieto L, Quesada JF, Cambero O, et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertility and Sterility. 2012 July;981:126–30. https://doi.org/10.1016/j.fertnstert.2012.03.052.
Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reproductive Toxicology. 2013 December;42:116–24. https://doi.org/10.1016/j.reprotox.2013.08.005.
Li K, Jiang S, Burn M, Kamal R. Is Elective Soft Tissue Hand Surgery Associated with Periprosthetic Joint Infection after Total Joint Arthroplasty? CLINICAL ORTHOPAEDICS AND RELATED RESEARCH. 2019 October;47710:2332–41. https://doi.org/10.1097/CORR.0000000000000801.
Sanchez AM, Papaleo E, Corti L, et al. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Human Reproduction. 2014 March 1;293:577–83. https://doi.org/10.1093/humrep/det466.
Aboulghar M. Anti-mullerian hormone in the management of infertility. Middle East Fertility Society Journal. 2014 March;191:1–7. https://doi.org/10.1016/j.mefs.2014.03.002.
Morin SJ, Patounakis G, Juneau CR, Neal SA, Scott RT, Seli E. Diminished ovarian reserve and poor response to stimulation in patients <38 years old: a quantitative but not qualitative reduction in per-formance. Human Reproduction. 2018 August 1;338:1489–98. https://doi.org/10.1093/humrep/dey238.
Irez T, Ocal P, Guralp O, Cetin M, Aydogan B, Sahmay S. Different serum anti-Müllerian hormone concentrations are associated with oocyte quality, embryo development parameters and IVF-ICSI outcomes. Arch Gynecol Obstet. 2011 November;2845:1295–301. https://doi.org/10.1007/s00404-011-1979-6.
Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age. JAMA. 2017 October 10;31814:1367. https://doi.org/10.1001/jama.2017.14588.
Gilchrist RB, Luciano AM, Richani D, et al. Oocyte maturation and quality: role of cyclic nucleo-tides. Reproduction. 2016 November;1525:R143-157. PMID: 27422885. https://doi.org/10.1530/REP-15-0606.
Singer ST, Vichinsky EP, Gildengorin G, van Disseldorp J, Rosen M, Cedars MI. Reproductive capac-ity in iron overloaded women with thalassemia major. Blood. 2011 September 8;11810:2878–81. PMID: 21757620. https://doi.org/10.1182/blood-2011-06-360271.
Uysal A, Alkan G, Kurtoğlu A, Erol O, Kurtoğlu E. Diminished ovarian reserve in women with transfusion-dependent beta-thalassemia major: Is iron gonadotoxic? Eur J Obstet Gynecol Reprod Biol. 2017 September;216:69–73. PMID: 28732253. https://doi.org/10.1016/j.ejogrb.2017.06.038.
Reubinoff BE, Har-El R, Kitrossky N, et al. Increased levels of redox-active iron in follicular fluid: a possible cause of free radical-mediated infertility in beta-thalassemia major. Am J Obstet Gynecol. 1996 March;1743:914–8. PMID: 8633668. https://doi.org/10.1016/s0002-9378(96)70325-3.
Roussou P, Tsagarakis NJ, Kountouras D, Livadas S, Diamanti-Kandarakis E. Beta-thalassemia ma-jor and female fertility: the role of iron and iron-induced oxidative stress. Anemia. 2013;2013:617204. PMID: 24396593. https://doi.org/10.1155/2013/617204.
Mensi L, Borroni R, Reschini M, et al. Oocyte quality in women with thalassaemia major: insights from IVF cycles. Eur J Obstet Gynecol Reprod Biol X. 2019 July;3:100048. PMID: 31404374. https://doi.org/10.1016/j.eurox.2019.100048.
Tyan PI, Radwan AH, Eid A, Haddad AG, Wehbe D, Taher AT. Novel approach to reactive oxygen species in nontransfusion-dependent thalassemia. Biomed Res Int. 2014;2014:350432. PMID: 25121095. https://doi.org/10.1155/2014/350432.
Decanter C, Robin G, Mailliez A, et al. Prospective assessment of follicular growth and the oocyte cohort after ovarian stimulation for fertility preservation in 90 cancer patients versus 180 matched controls. Reprod Biomed Online. 2018 May;365:543–51. PMID: 29506861. https://doi.org/10.1016/j.rbmo.2018.01.016.
Johnson LNC, Dillon KE, Sammel MD, et al. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod Biomed Online. 2013 April;264:337–44. PMID: 23415997. https://doi.org/10.1016/j.rbmo.2013.01.003.
Fabiani C, Ferrante MG, Meneghini C, et al. Female fertility preservation: Impact of cancer on ovarian function and oocyte quality. International Journal of Gynecology & Obstetrics. 2022;1561:166–71. https://doi.org/10.1002/ijgo.13702.
Hickman LC, Llarena NC, Valentine LN, Liu X, Falcone T. Preservation of gonadal function in women undergoing chemotherapy: a systematic review and meta-analysis of the potential role for gonadotropin-releasing hormone agonists. J Assist Reprod Genet. 2018 April;354:571–81. PMID: 29470701. https://doi.org/10.1007/s10815-018-1128-2.
Lambertini M, Moore HCF, Leonard RCF, et al. Gonadotropin-Releasing Hormone Agonists During Chemotherapy for Preservation of Ovarian Function and Fertility in Premenopausal Patients With Early Breast Cancer: A Systematic Review and Meta-Analysis of Individual Patient-Level Data. J Clin Oncol. 2018 July 1;3619:1981–90. PMID: 29718793. https://doi.org/10.1200/JCO.2018.78.0858.
Demeestere I, Basso O, Moffa F, Peccatori F, Poirot C, Shalom-Paz E. Fertility preservation in female cancer patients. Obstet Gynecol Int. 2012;2012:695041. PMID: 23125860. https://doi.org/10.1155/2012/695041.
Roness H, Gavish Z, Cohen Y, Meirow D. Ovarian follicle burnout: a universal phenomenon? Cell Cycle. 2013 October 15;1220:3245–6. PMID: 24036538. https://doi.org/10.4161/cc.26358.
Sofiyeva N, Siepmann T, Barlinn K, Seli E, Ata B. Gonadotropin-Releasing Hormone Analogs for Gonadal Protection During Gonadotoxic Chemotherapy: A Systematic Review and Meta-Analysis. Reprod Sci. 2019 July;267:939–53. PMID: 30270741. https://doi.org/10.1177/1933719118799203.
Elgindy E, Sibai H, Abdelghani A, Mostafa M. Protecting Ovaries During Chemotherapy Through Gonad Suppression: A Systematic Review and Meta-analysis. Obstet Gynecol. 2015 July;1261:187–95. PMID: 26241272. https://doi.org/10.1097/AOG.0000000000000905.
Brayboy LM, Oulhen N, Witmyer J, Robins J, Carson S, Wessel GM. Multidrug-resistant transport activity protects oocytes from chemotherapeutic agents and changes during oocyte maturation. Fertil Steril. 2013 November;1005:1428–35. PMID: 23953328. https://doi.org/10.1016/j.fertnstert.2013.07.002.
Kano M, Sosulski AE, Zhang L, et al. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc Natl Acad Sci U S A. 2017 February 28;1149:E1688–97. PMID: 28137855. https://doi.org/10.1073/pnas.1620729114.
Practice Committees of the American Society for Reproductive Medicine and the Society for As-sisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013 January;991:37–43. PMID: 23083924. https://doi.org/10.1016/j.fertnstert.2012.09.028.
Del-Pozo-Lérida S, Salvador C, Martínez-Soler F, Tortosa A, Perucho M, Giménez-Bonafé P. Preser-vation of fertility in patients with cancer (Review). Oncol Rep. 2019 May;415:2607–14. PMID: 30896846. https://doi.org/10.3892/or.2019.7063.
Mohsenzadeh M, Tabibnejad N, Vatanparast M, Anbari F, Ali Khalili M, Karimi-Zarchi M. Vitrifica-tion has detrimental effects on maturation, viability, and subcellular quality of oocytes post IVM in cancerous women: An experimental study. Int J Reprod Biomed. 2019 March;173:ijrm.v17i3.4516. PMID: 31435595. https://doi.org/10.18502/ijrm.v17i3.4516.
Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. JCO. 2006 June 20;2418:2917–31. https://doi.org/10.1200/JCO.2006.06.5888.
Noyes N, Knopman JM, Long K, Coletta JM, Abu-Rustum NR. Fertility considerations in the man-agement of gynecologic malignancies. Gynecologic Oncology. 2011 March;1203:326–33. https://doi.org/10.1016/j.ygyno.2010.09.012.
Clough KB, Goffinet F, Labib A, et al. Laparoscopic unilateral ovarian transposition prior to irra-diation: Prospective study of 20 cases. Cancer. 1996 June 15;7712:2638–45. https://doi.org/10.1002/(SICI)1097-0142(19960615)77:12<2638::AID-CNCR30>3.0.CO;2-R.
Visvanathan D. A new technique of laparoscopic ovariopexy before irradiation. Fertility and Ste-rility. 2003 May;795:1204–6. https://doi.org/10.1016/S0015-0282(03)00157-2.
McKenzie LJ, Pangas SA, Carson SA, et al. Human cumulus granulosa cell gene expression: a pre-dictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004 Decem-ber;1912:2869–74. PMID: 15471935. https://doi.org/10.1093/humrep/deh535.
Assidi M, Montag M, Van der Ven K, Sirard M-A. Biomarkers of human oocyte developmental competence expressed in cumulus cells before ICSI: a preliminary study. J Assist Reprod Genet. 2011 February;282:173–88. PMID: 20953827. https://doi.org/10.1007/s10815-010-9491-7.
Palomba S, Daolio J, La Sala GB. Oocyte Competence in Women with Polycystic Ovary Syndrome. Trends Endocrinol Metab. 2017 March;283:186–98. PMID: 27988256. https://doi.org/10.1016/j.tem.2016.11.008.
Gohari taban S, Amiri I, Saidijam M, et al. ADAMTS proteoglycanases downregulation with im-paired oocyte quality in PCOS. Archives of Endocrinology and Metabolism. 2021 January 18. https://doi.org/10.20945/2359-3997000000321.
Nikbakht R, Mohammadjafari R, Rajabalipour M, Moghadam MT. Evaluation of oocyte quality in Polycystic ovary syndrome patients undergoing ART cycles. Fertil Res and Pract. 2021 Decem-ber;71:2. https://doi.org/10.1186/s40738-020-00094-z.
Sigala J, Sifer C, Dewailly D, et al. Is polycystic ovarian morphology related to a poor oocyte quali-ty after controlled ovarian hyperstimulation for intracytoplasmic sperm injection? Results from a prospective, comparative study. Fertil Steril. 2015 January;1031:112–8. PMID: 25450303. https://doi.org/10.1016/j.fertnstert.2014.09.040.
Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018 July 19;41:9. PMID: 30026507. https://doi.org/10.1038/s41572-018-0008-5.
Smolarz B, Szyłło K, Romanowicz H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int J Mol Sci. 2021 September 29;2219:10554. PMID: 34638893. https://doi.org/10.3390/ijms221910554.
Cheraghi E, Mehranjani MS, Shariatzadeh MA, Esfahani MHN, Ebrahimi Z. N-Acetylcysteine im-proves oocyte and embryo quality in polycystic ovary syndrome patients undergoing intracytoplas-mic sperm injection: an alternative to Metformin. Reprod Fertil Dev. 2016 April;286:723–31. PMID: 25482371. https://doi.org/10.1071/RD14182.
Regidor P-A, Schindler AE, Lesoine B, Druckman R. Management of women with PCOS using myo-inositol and folic acid. New clinical data and review of the literature. Horm Mol Biol Clin Investig. 2018 March 2;342:/j/hmbci.2018.34.issue-2/hmbci-2017-0067/hmbci-2017-0067.xml. PMID: 29498933. https://doi.org/10.1515/hmbci-2017-0067.
Akbari Sene A, Tabatabaie A, Nikniaz H, et al. The myo-inositol effect on the oocyte quality and fertilization rate among women with polycystic ovary syndrome undergoing assisted reproductive technology cycles: a randomized clinical trial. Arch Gynecol Obstet. 2019 June;2996:1701–7. PMID: 30919036. https://doi.org/10.1007/s00404-019-05111-1.
Pacchiarotti A, Carlomagno G, Antonini G, Pacchiarotti A. Effect of myo-inositol and Melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol. 2016;321:69–73. PMID: 26507336. https://doi.org/10.3109/09513590.2015.1101444.
Mendoza N, Galan MI, Molina C, et al. High dose of d-chiro-inositol improves oocyte quality in women with polycystic ovary syndrome undergoing ICSI: a randomized controlled trial. Gynecol Endocrinol. 2020 May;365:398–401. PMID: 31657275. https://doi.org/10.1080/09513590.2019.1681959.
Gong Y, Luo S, Fan P, et al. Growth hormone alleviates oxidative stress and improves oocyte quali-ty in Chinese women with polycystic ovary syndrome: a randomized controlled trial. Sci Rep. 2020 October 30;101:18769. PMID: 33127971. https://doi.org/10.1038/s41598-020-75107-4.
Tesarik J, Galán-Lázaro M, Conde-López C, Chiara-Rapisarda AM, Mendoza-Tesarik R. The Effect of GH Administration on Oocyte and Zygote Quality in Young Women With Repeated Implantation Failure After IVF. Front Endocrinol (Lausanne). 2020;11:519572. PMID: 33117271. https://doi.org/10.3389/fendo.2020.519572.
Narkwichean A, Maalouf W, Baumgarten M, et al. Efficacy of Dehydroepiandrosterone (DHEA) to overcome the effect of ovarian ageing (DITTO): A proof of principle double blinded randomized pla-cebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2017 November;218:39–48. PMID: 28934714. https://doi.org/10.1016/j.ejogrb.2017.09.006.
Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplemen-tation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000 Octo-ber;1510:2129–32. PMID: 11006185. https://doi.org/10.1093/humrep/15.10.2129.
Hillier SG, De Zwart FA. Evidence that granulosa cell aromatase induction/activation by follicle-stimulating hormone is an androgen receptor-regulated process in-vitro. Endocrinology. 1981 Oc-tober;1094:1303–5. PMID: 6793349. https://doi.org/10.1210/endo-109-4-1303.
Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicu-lar growth in the primate ovary. J Clin Invest. 1998 June 15;10112:2622–9. PMID: 9637695. https://doi.org/10.1172/JCI2081.
Baart EB, Martini E, Eijkemans MJ, et al. Milder ovarian stimulation for in-vitro fertilization re-duces aneuploidy in the human pre-implantation embryo: a randomized controlled trial. Hum Re-prod. 2007 April;224:980–8. PMID: 17204525. https://doi.org/10.1093/humrep/del484.
Karasu Y, Dilbaz B, Demir B, et al. REPRODUCTIVE ENDOCRINOLOGY. Human Reproduction. 2012 January 1;27suppl 2:ii302–37. https://doi.org/10.1093/humrep/27.s2.88.
Balasch J, Fábregues F, Peñarrubia J, et al. Pretreatment with transdermal testosterone may im-prove ovarian response to gonadotrophins in poor-responder IVF patients with normal basal con-centrations of FSH. Hum Reprod. 2006 July;217:1884–93. PMID: 16517559. https://doi.org/10.1093/humrep/del052.
Yovich JL, Stanger JD. Growth hormone supplementation improves implantation and preg-nancy productivity rates for poor-prognosis patients undertaking IVF. Reproductive BioMedicine Online. 2010 July;211:37–49. https://doi.org/10.1016/j.rbmo.2010.03.013.
Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo-inositol plus Melato-nin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol. 2011 Novem-ber;2711:857–61. PMID: 21463230. https://doi.org/10.3109/09513590.2011.564687.
Babayev E, Seli E. Oocyte mitochondrial function and reproduction. Curr Opin Obstet Gynecol. 2015 June;273:175–81. PMID: 25719756. https://doi.org/10.1097/GCO.0000000000000164.
Fakih MH. The AUGMENTSM Treatment: Physician Reported Outcomes of the Initial Global Pa-tient Experience. JFIV Reprod Med Genet. 2015;0303. https://doi.org/10.4172/2375-4508.1000154.
Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013 October;3438:2949–3003. PMID: 23996286. https://doi.org/10.1093/eurheartj/eht296.
El Shourbagy SH, Spikings EC, Freitas M, St John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006 February;1312:233–45. PMID: 16452717. https://doi.org/10.1530/rep.1.00551.
Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril. 2011 January;951:64–7. PMID: 20688325. https://doi.org/10.1016/j.fertnstert.2010.06.064.
Niu Z, Lin N, Gu R, Sun Y, Feng Y. Associations Between Insulin Resistance, Free Fatty Acids, and Oocyte Quality in Polycystic Ovary Syndrome During In Vitro Fertilization. The Journal of Clinical Endocrinology & Metabolism. 2014 November 1;9911:E2269–76. https://doi.org/10.1210/jc.2013-3942.
Saharkhiz N, Zamaniyan M, Salehpour S, et al. A comparative study of dydrogesterone and mi-cronized progesterone for luteal phase support during in vitro fertilization (IVF) cycles. Gynecol En-docrinol. 2016;323:213–7. PMID: 26486011. https://doi.org/10.3109/09513590.2015.1110136.
Richter TA, Robinson JE, Lozano JM, Evans NP. Progesterone can block the preovulatory gonado-tropin-releasing hormone/luteinising hormone surge in the ewe by a direct inhibitory action on oes-tradiol-responsive cells within the hypothalamus. J Neuroendocrinol. 2005 March;173:161–9. PMID: 15796768. https://doi.org/10.1111/j.1365-2826.2005.01287.x.
Kuang Y, Chen Q, Fu Y, et al. Medroxyprogesterone acetate is an effective oral alternative for pre-venting premature luteinizing hormone surges in women undergoing controlled ovarian hyperstim-ulation for in vitro fertilization. Fertil Steril. 2015 July;1041:62-70.e3. PMID: 25956370. https://doi.org/10.1016/j.fertnstert.2015.03.022.
Zhu X, Ye H, Fu Y. Duphaston and human menopausal gonadotropin protocol in normally ovu-latory women undergoing controlled ovarian hyperstimulation during in vitro fertiliza-tion/intracytoplasmic sperm injection treatments in combination with embryo cryopreservation. Fertil Steril. 2017 September;1083:505-512.e2. PMID: 28697910. https://doi.org/10.1016/j.fertnstert.2017.06.017.
Zavareh S, Saberivand A, Salehnia M. The Effect of Progesterone on the In vitro Maturation and Developmental Competence of Mouse Germinal Vesicle Oocytes. Int J Fertil Steril. 2009 May;31. https://doi.org/10.22074/ijfs.2009.45742.
Liu D, Mo G, Tao Y, Wang H, Liu XJ. Putrescine supplementation during in vitro maturation of aged mouse oocytes improves the quality of blastocysts. Reprod Fertil Dev. 2017 July;297:1392–400. PMID: 27319359. https://doi.org/10.1071/RD16061.
Ben-Meir A, Burstein E, Borrego-Alvarez A, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015 October;145:887–95. PMID: 26111777. https://doi.org/10.1111/acel.12368.
Deswal R, Narwal V, Dang A, Pundir CS. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J Hum Reprod Sci. 2020 December;134:261–71. PMID: 33627974. https://doi.org/10.4103/jhrs.JHRS_95_18.
Published
How to Cite
Issue
Section
Copyright (c) 2022 International Journal of Clinical Research

This work is licensed under a Creative Commons Attribution 4.0 International License.